How Many Valence Electrons Does Oxygen Have? Unveiled!

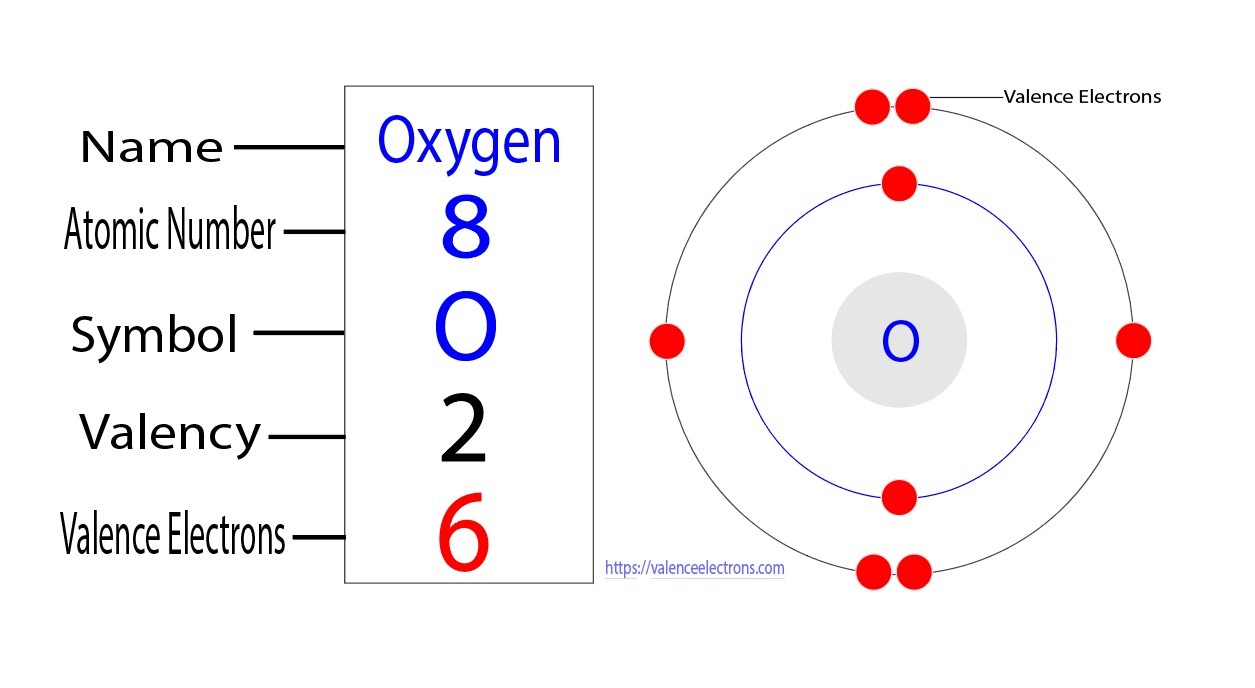
Oxygen has 6 valence electrons. This determines its reactivity and bonding capabilities.
Oxygen, a vital element in the periodic table, possesses 6 valence electrons. These electrons play a crucial role in oxygen’s ability to form various chemical bonds with other elements. Understanding the number of valence electrons in oxygen is essential for predicting its behavior in chemical reactions and its role in supporting life processes.
In nature, oxygen commonly forms bonds with hydrogen to create water molecules, showcasing the significance of its valence electrons in fostering essential interactions. Exploring the properties and functions of oxygen’s valence electrons provides valuable insights into its fundamental role in the natural world and the diverse chemical reactions that sustain life.
Credit: praxilabs.com
The Atomic Structure Of Oxygen
The Significance Of Electrons
Oxygen has 6 valence electrons, which are crucial for its chemical behavior.
- Valence electrons determine an element’s reactivity.
- They participate in chemical bonding.
- Their arrangement affects an atom’s stability.
Shells And Orbitals
Oxygen’s electrons are distributed in 2 shells: the first shell holds 2 electrons, while the second shell holds 4.
The second shell has 2 subshells, with 1 containing 2 electrons and the other containing 4.
Valence Electrons: A Primer
Oxygen has six valence electrons, making it a crucial element for various chemical reactions and bonding processes. Understanding the role of valence electrons in oxygen’s reactivity is fundamental in chemistry studies and practical applications.
Understanding the concept of valence electrons is crucial when it comes to comprehending the behavior of elements in chemical reactions. Valence electrons are the electrons found in the outermost energy level, or shell, of an atom. They play a significant role in the formation of chemical bonds between atoms, determining an element’s reactivity and its ability to combine with other elements.
Defining Valence Electrons
Valence electrons can be defined as the electrons located in the outermost energy level of an atom. These electrons are the ones involved in chemical reactions, as they are more likely to interact with other atoms. The number of valence electrons an atom possesses is determined by its position in the periodic table. For example, oxygen, which is found in Group 16 of the periodic table, has six valence electrons.
Role In Chemical Bonding
Valence electrons play a crucial role in chemical bonding, as they are responsible for the formation of bonds between atoms. Atoms with incomplete outer energy levels tend to gain, lose, or share electrons in order to achieve a stable electron configuration, usually with eight valence electrons. This is known as the octet rule. For instance, oxygen, with six valence electrons, can form bonds by either gaining two electrons to complete its outer energy level or sharing two electrons with another atom.
By gaining two electrons, oxygen can form an ionic bond with elements that tend to lose electrons, such as metals. On the other hand, by sharing two electrons, oxygen can form a covalent bond with elements that also require two more electrons to complete their outer energy levels. These chemical bonds allow atoms to achieve a more stable configuration and result in the formation of compounds with unique properties.
In conclusion, valence electrons are the key players in chemical reactions and bonding between atoms. By understanding the concept of valence electrons and their role in chemical bonding, we can gain insights into the behavior and reactivity of elements. This knowledge is fundamental in various fields, including chemistry, materials science, and biology.
Determining Oxygen’s Valence Electrons
Oxygen has six valence electrons, making it a key player in chemical bonding and reactions. Understanding its valence electron configuration is crucial for predicting its behavior in various chemical compounds.
Electronic Configuration
Oxygen, a chemical element with the symbol O and atomic number 8, is an essential element for life on Earth. It plays a crucial role in respiration and the formation of water. One key aspect of oxygen’s chemical behavior is its valence electrons, which are the electrons in the outermost energy level of an atom. Determining the number of valence electrons in oxygen is important for understanding its chemical properties and reactivity. To find the number of valence electrons in oxygen, we need to look at its electronic configuration. The electronic configuration of an element represents how its electrons are distributed among the various energy levels or orbitals. In the case of oxygen, its electronic configuration is 1s^2 2s^2 2p^4. This configuration tells us that oxygen has a total of eight electrons. The first energy level (1s) can hold a maximum of two electrons, the second energy level (2s) can hold a maximum of eight electrons, and the third energy level (2p) can hold a maximum of six electrons. Since oxygen has only two energy levels, we focus on the electrons in the outermost energy level, which is the second energy level. In oxygen’s case, the second energy level has a total of six electrons distributed among the 2s and 2p orbitals. The 2s orbital can hold a maximum of two electrons, and the 2p orbital can hold a maximum of six electrons. Oxygen has two electrons in the 2s orbital and four electrons in the 2p orbital. Therefore, oxygen has six valence electrons.
Using The Periodic Table
The periodic table is a valuable tool for determining the number of valence electrons in an element. Each element’s position on the periodic table can provide information about its electron configuration and the number of valence electrons it possesses. To determine the number of valence electrons in oxygen using the periodic table, we can look at its group number. Oxygen belongs to Group 16, also known as the Oxygen Group or the Chalcogens. Elements in Group 16 have six valence electrons, which can be seen by their position in the sixth column of the periodic table. By referring to the periodic table, we can quickly determine that oxygen has six valence electrons, confirming the information obtained from its electronic configuration. In conclusion, oxygen has six valence electrons. Understanding the number of valence electrons is crucial for predicting the chemical behavior of oxygen and its reactivity with other elements. By knowing the electronic configuration and using the periodic table, we can easily determine the number of valence electrons in oxygen, contributing to our understanding of its chemical properties.
Oxygen’s Place In The Periodic Table
Group And Period Insights
Oxygen is found in Group 16 and Period 2 of the periodic table. In Group 16, it is one of the chalcogens, also known as the oxygen family. This group is known for its diverse chemical properties, and oxygen, with its high electronegativity, plays a crucial role in the formation of various compounds. As for its position in Period 2, oxygen is located among the elements with the fewest valence electrons, which affects its reactivity and bonding behavior.
Comparing With Other Elements
When comparing oxygen with other elements in the periodic table, it stands out for its unique characteristics. Unlike the noble gases in Group 18, oxygen readily forms compounds due to its six valence electrons. In contrast, elements in Group 1, such as hydrogen and lithium, possess only one valence electron, leading to different chemical behaviors. Additionally, when compared to its neighbor, nitrogen, oxygen has a higher electronegativity, influencing its ability to attract electrons in chemical reactions.
The Octet Rule And Oxygen
Oxygen has 6 valence electrons, following the Octet Rule. This makes it highly reactive, often forming bonds with other elements to complete its outer shell.
Oxygen, with its atomic number of 8, is a crucial element in our atmosphere and plays a vital role in various chemical reactions. To understand its behavior, we must delve into the concept of the octet rule.
Fulfilling The Octet
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. In the case of oxygen, it has six valence electrons, leaving it two electrons short of a full octet.
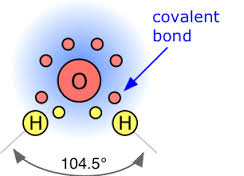
To fulfill the octet, oxygen can either gain two electrons or share electrons with other atoms. Gaining two electrons would result in a negative charge, turning oxygen into an oxide ion (O2-). This ion is commonly found in compounds such as water (H2O) and carbon dioxide (CO2), where oxygen forms bonds with other elements.
On the other hand, oxygen can also share electrons by forming covalent bonds. Each covalent bond involves the sharing of one electron pair. Oxygen can form double bonds, where it shares two pairs of electrons, or even triple bonds in certain compounds.
Exceptions And Variations
While the octet rule serves as a useful guideline, there are exceptions and variations to consider. Some elements, including oxygen, can expand their valence shell beyond eight electrons. This occurs when oxygen forms compounds with elements from the third period or below, such as sulfur or phosphorus.
Additionally, there are cases where oxygen doesn’t fulfill the octet. For example, in ozone (O3), oxygen forms a double bond with one oxygen atom and a single bond with another, resulting in an electron configuration with three shared pairs and one unshared pair of electrons.
In summary, oxygen typically follows the octet rule by either gaining or sharing electrons to achieve a stable electron configuration. However, exceptions and variations exist, allowing for a deeper understanding of oxygen’s role in chemical reactions.
Credit: socratic.org
Oxygen’s Valency Vs. Valence Electrons
Defining Valency
Valency represents the combining capacity of an element, indicating the number of bonds it can form with other atoms. It is determined by the number of electrons an atom needs to gain, lose, or share to achieve a stable configuration.
How Valency Differs From Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom, while valency is the ability of an atom to combine with other atoms. Oxygen has 6 valence electrons and a valency of 2, meaning it can gain 2 electrons to achieve a stable octet configuration.
Implications In Chemical Reactions
Oxygen’s valence electrons impact chemical reactions significantly.
Oxygen In Covalent Bonds
Oxygen forms covalent bonds by sharing electrons.
Ionic Bonds And Oxidation States
Oxygen can gain or lose electrons in ionic bonds.

Credit: www.youtube.com
Real-world Applications
Oxygen plays a crucial role in various real-world applications. Let’s explore how oxygen’s valence electrons are utilized in different fields.
Importance In Organic Compounds
Oxygen valence electrons are essential in forming organic compounds.
- Facilitates the formation of hydrogen bonds in organic molecules.
- Enables the creation of ester and ether functional groups.
- Supports the synthesis of alcohols and carbonyl compounds.
Oxygen In Industrial Processes
Oxygen’s valence electrons are integral in various industrial applications.
- Used in metallurgy to enhance oxidation reactions.
- Plays a vital role in wastewater treatment processes.
- Utilized in chemical synthesis for producing various compounds.
Frequently Asked Questions
Q: How Many Valence Electrons Does Oxygen Have?
A: Oxygen has six valence electrons, which are located in its outermost shell. These electrons are crucial in forming chemical bonds with other atoms to create molecules.
Q: Why Is The Number Of Valence Electrons Important For Oxygen?
A: The number of valence electrons in oxygen determines how it reacts with other elements. Because it has six valence electrons, oxygen can form up to two covalent bonds with other atoms to create molecules.
Q: What Happens When Oxygen Loses Or Gains An Electron?
A: If oxygen loses an electron, it becomes a positively charged ion, known as an oxidizing agent. If it gains an electron, it becomes a negatively charged ion, known as a reducing agent.
Q: How Does Oxygen’s Valence Electron Configuration Affect Its Reactivity?
A: Oxygen’s valence electron configuration makes it highly reactive, as it is constantly seeking to fill its outermost shell. This reactivity allows it to form strong bonds with other elements, making it an important component in many chemical reactions.
Conclusion
Oxygen has six valence electrons, which means it requires two more electrons to achieve a full outer shell. Understanding the number of valence electrons an atom has is essential in predicting its chemical properties and reactivity. By knowing the electron configuration of oxygen, scientists can predict its bonding behavior and its role in various chemical reactions.
Knowing this information is crucial in the fields of chemistry, physics, and material science.



